Abstract
Introduction: Vasoactive intestinal polypeptide (VIP) is an immunosuppressive neuropeptide. Activation of donor T cells leading to graft-versus-host disease (GvHD) in allogeneic bone marrow transplant (allo-BMT) occurs via early post transplant interactions between donor T cells and host antigen presenting cells and leads to upregulation of immune co-inhibitory pathways, including expression of VIP in T cells and dendritic cells. We have previously shown VIP signaling favorably regulates the activation status and enhances the graft-versus-leukemia (GvL) activities of donor T cells (Li et al., Cancer Research 2016). The role of the VIP produced by host antigen presenting cells in regulating GvHD in allo-BMT is unknown. We tested the hypothesis that absence of VIP expression by host cells would augment donor T cell activation and increase GvHD.
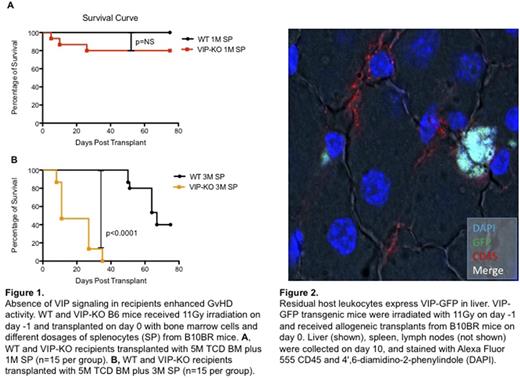
Methods and Results: We used the B10BR->B6 MHC fully mismatched murine model of allogeneic BMT. Recipient wildtype (WT) and VIP knockout B6 (VIP-KO) mice were lethally irradiated with 11Gy and injected i.v. with 5×106 T cell depleted (TCD) bone marrow cells (BM) plus 0, 1 or 3 ×106 splenocytes from WT B10BR donors. Fifteen mice (males and females) were assigned to each splenocytes dosage group. The survival of recipients after BMT was recorded daily and the average clinical GvHD scores were obtained by combining weight-loss, activity, posture, fur texture and skin integrity data following standard published procedures. All three groups of WT recipients had better survival than VIP-KO recipients. VIP-KO mice transplanted with TCD BM alone had only 33.3% survival at day 75 compared with 73.3% survival among the WT recipients. Chimerism studies on day 10 post-BMT showed equivalent level of donor chimerism: 80% donor in WT recipients vs 72% donor in VIP-KO recipients, indicating increased mortality in the VIP-KO recipients was not due to early graft rejection. The addition of donor splenocytes to the graft resulted in significantly more GvHD-related mortality in VIP-KO recipients compared with WT recipients (Figure 1), with 80% vs 100% day 75 survival among recipients of 1x106 splenocytes (p=NS) and 0% vs 40% survival (p<0.01) among recipients of 3x106 donor splenocytes, respectively.
To determine how the absence of VIP production in KO recipients modulates immune effector mechanisms that contributes to the differential survival rate post allo-BMT, we analyzed the recipients' splenocytes on day 30 post transplant with flow cytometry and staining with CD3, CD4, CD8, CD62L, CD44 and PD1 antibodies. VIP-KO recipients transplanted with 5M TCD BM plus 1M splenocytes had more CD4+ central memory and effector T cells, and CD8+ central memory and effector T cells. VIP-KO recipients transplanted with 5×106 TCD BM alone in addition had significantly more CD4 and CD8 effector T cells (p<0.01). VIP-KO recipients transplanted with 5×106 TCD BM plus 1x106 splenocytes had more PD1 expression on donor CD4+ central memory and effector T cells, and CD8+ central memory effector T cells, consistent with enhanced antigen-mediated activation.
To visualize VIP produced by the host cells, we transplanted C57/BL6 recipients expressing a GFP transgene under the control of the VIP promoter (n=10) with 5×106 TCD BM plus 3 ×106 splenocytes from WT B10BR donors, and visualized VIP gene promoter activity as GFP expression in recipient lymphoid tissues and GvHD target organs. Only small number of CD45+ VIP-GFP+ cells were detected from in host liver, spleen and lymph nodes on day 10 post transplant (Figure 2), suggesting residual host leukocytes, particularly dendritic cells, expressed VIP.
Conclusion: VIP production by recipient antigen presenting cells is a key pathway in limiting the activation of donor T cells. Physiological expression of VIP by host leukocytes regulate the GvHD activity of donor T cells. Novel methods to enhance VIP production in transplant recipients or the parenteral administration of VIP receptor agonists may limit GvHD, and may be particularly important in allo-BMT where GvL is not necessarily critical for success, such as transplants for hemoglobinopathy.
Waller: Novartis Pharmaceuticals Corporation: Consultancy, Honoraria, Research Funding; PRA: Consultancy; AMGEN: Consultancy; National Institutes of Health: Research Funding; Coulter Foundation: Research Funding; Helocyte: Consultancy; Cambium Medical Technologies: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; Katz Foundation: Research Funding; Chimerix: Equity Ownership; Cerus: Equity Ownership; Celldex: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal